The Results of Dehydration Reactions Can Be Reversed by
2.4C: Hydrolysis
- Page ID
- 12684
Hydrolysis reactions effect in the breakdown of polymers into monomers by using a h2o molecule and an enzymatic catalyst.
Learning Objectives
- Explicate hydrolysis reactions
Primal Points
- Hydrolysis reactions utilize water to breakdown polymers into monomers and is the opposite of dehydration synthesis, which forms water when synthesizing a polymer from monomers.
- Hydrolysis reactions intermission bonds and release energy.
- Biological macromolecules are ingested and hydrolyzed in the digestive tract to form smaller molecules that can be absorbed by cells and and so further broken down to release free energy.
Primal Terms
- enzyme: a globular protein that catalyses a biological chemical reaction
- hydrolysis: A chemical process of decomposition involving the splitting of a bond by the add-on of water.
Hydrolysis
Polymers are cleaved downward into monomers in a process known as hydrolysis, which means "to dissever h2o," a reaction in which a water molecule is used during the breakup. During these reactions, the polymer is cleaved into two components. If the components are un-ionized, ane part gains a hydrogen atom (H-) and the other gains a hydroxyl group (OH–) from a split water molecule. This is what happens when monosaccharides are released from circuitous carbohydrates via hydrolysis.
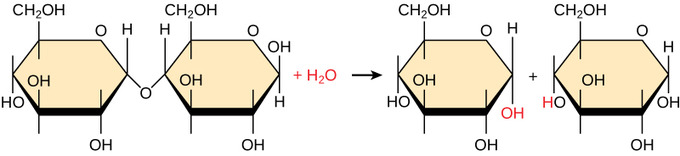
If the components are ionized after the split, one part gains 2 hydrogen atoms and a positive charge, the other role gains an oxygen atom and a negative charge. This is what happens when amino acids are released from protein chains via hydrolysis.

These reactions are in dissimilarity to dehydration synthesis (also known equally condensation) reactions. In aridity synthesis reactions, a h2o molecule is formed equally a upshot of generating a covalent bond betwixt ii monomeric components in a larger polymer. In hydrolysis reactions, a water molecule is consumed as a result of breaking the covalent bond property together two components of a polymer.
Dehydration and hydrolysis reactions are chemical reactions that are catalyzed, or "sped upwardly," past specific enzymes; aridity reactions involve the germination of new bonds, requiring free energy, while hydrolysis reactions pause bonds and release energy.
In our bodies, nutrient is first hydrolyzed, or broken downwardly, into smaller molecules by catalytic enzymes in the digestive tract. This allows for easy absorption of nutrients by cells in the intestine. Each macromolecule is cleaved downwards by a specific enzyme. For example, carbohydrates are broken downwards past amylase, sucrase, lactase, or maltase. Proteins are broken downwardly past the enzymes trypsin, pepsin, peptidase and others. Lipids are broken down past lipases. Once the smaller metabolites that result from these hydrolytic enzymezes are absorbed past cells in the torso, they are further broken downward by other enzymes. The breakdown of these macromolecules is an overall energy-releasing procedure and provides energy for cellular activities.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided past: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- OpenStax Higher, Biological science. October xvi, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44395/latest...ol11448/latest . License: CC BY: Attribution
- OpenStax College, Synthesis of Biological Macromolecules. October 23, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44397/latest/ . License: CC Past: Attribution
- An Introduction to Molecular Biology/Macromolecules and Cells. Provided past: Wikibooks. Located at: en.wikibooks.org/wiki/An_Introduction_to_Molecular_Biology/Macromolecules_and_Cells . License: CC BY-SA: Attribution-ShareAlike
- Free High School Science Texts Project, Organic Macromolecules: Biological Macromolecules. October 23, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m39433/latest/ . License: CC BY: Attribution
- monomer. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/monomer . License: CC BY-SA: Attribution-ShareAlike
- polymer. Provided past: Wiktionary. Located at: en.wiktionary.org/wiki/polymer . License: CC By-SA: Attribution-ShareAlike
- OpenStax College, Introduction. October xvi, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44395/latest...e_03_00_01.jpg . License: CC BY: Attribution
- Sucrose-inkscape.svg.png. Provided by: Wikimedia. Located at: commons.wikimedia.org/wiki/File:Sucrose-inkscape.svg . License: CC BY-SA: Attribution-ShareAlike
- Building_blocks_of_life.png. Provided by: Wikimedia. Located at: https://upload.wikimedia.org/Wikipedia/commons/0/0a/Building_blocks_of_life.png . License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Biology. Oct 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44397/latest...ol11448/latest . License: CC By: Attribution
- monomer. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/monomer . License: CC By-SA: Attribution-ShareAlike
- covalent bond. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/covalent_bond . License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Introduction. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44395/latest...e_03_00_01.jpg . License: CC Past: Attribution
- Sucrose-inkscape.svg.png. Provided by: Wikimedia. Located at: eatables.wikimedia.org/wiki/File:Sucrose-inkscape.svg . License: CC By-SA: Attribution-ShareAlike
- Building_blocks_of_life.png. Provided by: Wikimedia. Located at: https://upload.wikimedia.org/Wikipedia/commons/0/0a/Building_blocks_of_life.png . License: CC BY-SA: Attribution-ShareAlike
- OpenStax Higher, Synthesis of Biological Macromolecules. October xvi, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44397/latest...e_03_01_01.jpg . License: CC Past: Attribution
- OpenStax Higher, Biology. Oct 16, 2013. Provided past: OpenStax CNX. Located at: http://cnx.org/content/m44397/latest...ol11448/latest . License: CC By: Attribution
- enzyme. Provided past: Wiktionary. Located at: en.wiktionary.org/wiki/enzyme . License: CC Past-SA: Attribution-ShareAlike
- hydrolysis. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/hydrolysis . License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Introduction. October sixteen, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44395/latest...e_03_00_01.jpg . License: CC By: Attribution
- Sucrose-inkscape.svg.png. Provided by: Wikimedia. Located at: https://commons.wikimedia.org/wiki/File:Sucrose-inkscape.svg . License: CC BY-SA: Attribution-ShareAlike
- Building_blocks_of_life.png. Provided by: Wikimedia. Located at: https://upload.wikimedia.org/Wikipedia/commons/0/0a/Building_blocks_of_life.png . License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Synthesis of Biological Macromolecules. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44397/latest...e_03_01_01.jpg . License: CC BY: Attribution
- OpenStax College, Synthesis of Biological Macromolecules. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44397/latest...e_03_01_02.jpg . License: CC BY: Attribution
Source: https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book:_General_Biology_%28Boundless%29/02:_The_Chemical_Foundation_of_Life/2.4:_Synthesis_of_Biological_Macromolecules/2.4C:_Hydrolysis
0 Response to "The Results of Dehydration Reactions Can Be Reversed by"
Postar um comentário